Background: Varnimcabtagene autoleucel (IMN-003A) is an autologous CD19 directed CAR-T cell product with a 4-1BB co-stimulatory domain and a non-FMC63 murine single chain variable fragment (A3B1 binder), manufactured in India, tested in the IMAGINE study (CTRI/2022/03/041162), a phase-2 clinical trial for patients (pts) with relapsed and/or refractory B cell malignancies. A fractionated infusion of 1 x 10 6 CAR+ cells (IMN-003A)/kg for B-ALL cohort and 5 x 10 6 CAR+ cells (IMN-003A)/kg for B-NHL cohort was administered over 3 days (10%, 30%, 60%). Phenotypic data comparing the apheresis (AP) and final product (FP) from B-ALL and B-NHL cohorts, related manufacturing and clinical response observations are discussed in this abstract.
Methods: IMN-003A was manufactured using the Miltenyi CliniMACS Prodigy®, a cGMP compliant closed system. The apheresis and final infusion product (FP) were analysed for percent CAR transduction and T cell subsets by flow cytometry. CAR copies in the final product were determined by ddPCR. Cell counts measured using automated Vi-cell counter were used to calculate fold expansion.
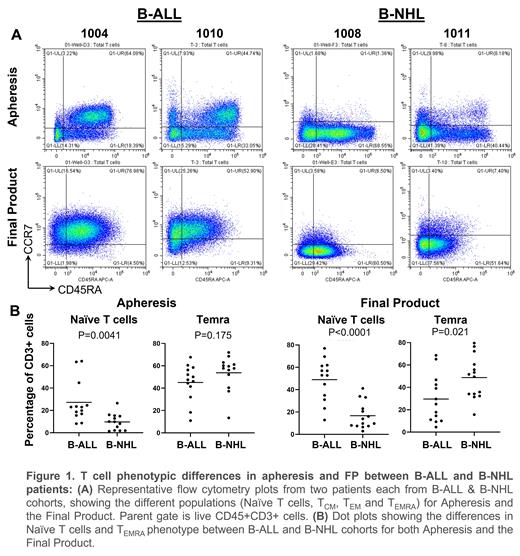
Results: Apheresis PBMC from 25 patients in the IMAGINE study were selected for T-cells, transduced & expanded to achieve the FP, which was infused in 24 patients (1 patient withdrawn). Three patients needed a second apheresis and re-manufacturing to achieve the target dose. The FP comprised primarily of CD3+ T cells (median 99.38%, range 96.3 - 99.92%) with mean of 38.18% CAR+ T cells for B-ALL cohort (range: 20.28 - 58.35%) and significantly lower 27.53% CAR+ T-cells for B-NHL cohort (range: 8.5 - 51.07%, p=0.02). The vector copy number (VCN) however did not differ between the two cohorts (mean of 2.66 and 2.37 copies for B-ALL and B-NHL cohorts respectively; p=0.4). The fold expansion at Day 6 of manufacturing was also significantly lower for B-NHL cohort (6.93) compared to the B-ALL cohort (10.33, p=0.02). Consistent with the relatively more challenging manufacturing for the B-NHL cohort with 5 times higher target dose, it was observed that the mean proportion of naïve T-cells (CCR7 + CD45RA +) was lower in both AP (B-NHL 9.71%, B-ALL 27.24%, p<0.01) and FP (B-NHL 16.68%, B-ALL 48.94%, p<0.0001), while the terminally differentiated T EMRA and effector memory T EM subsets were higher in the AP (B-NHL 53.8%, B-ALL 45.08%, p=0.175) & FP (B-NHL 48.77%, B-ALL 29.54%, p=0.021), highlighting the T-cell phenotypic differences in the two cohorts.
The mean CD4/CD8 ratio in AP & FP was 0.56 and 1.14 respectively. There was reversal of CD4/CD8 ratio from apheresis to final product (CAR+ T cells, ratio=3.37) which was statistically significant (p<0.01). The CD4:CD8 ratio did not differ between B-ALL and B-NHL cohorts. This could potentially be explained by the higher proportion of naïve T-cells observed within CD4 subset compared to CD8 subset as against the proportion of T EMRA cells. Despite the higher fraction of CD4 CAR-T cells within the final product infused, post-infusion, there was a statistically significant trend (p<0.01) towards reversal of CD4/CD8 ratio in the peripheral blood from D0 to D+90. Ongoing flow-cytometry based analysis shall help further characterise the CAR+ T-cells post-infusion.
Interestingly, despite phenotypic differences in T-cell subsets between B-ALL and B-NHL, overall response rate (ORR) at D+90 was very similar for both cohorts [B-ALL 80% (n=8/10); B-NHL 81.8% (9/11)]. Updated results shall be presented at the meeting.
Conclusions: Differences in the manufacturing outcomes (CAR % transduction, fold expansion) and T cell phenotype (naïve and effector T EMRA cell subsets) were observed in Apheresis and FP, between the B-ALL and B-NHL cohorts. A higher fraction of relatively more differentiated T cell phenotypes of effector (T EMRA) and effector memory (T EM) and a lower fraction of naïve T cells in the B-NHL cohort and within CD8 T cells, reflects a manufacturing conundrum for B-NHL patients with a higher target dose and the CD4:CD8 ratio in the final product. However, these differences in the final product did not adversely impact the clinical responses at D+90 with equivalent efficacy in both cohorts. The ideal T cell phenotype or their proportions within the final product for maximum efficacy thus remains uncertain. A deeper characterisation including exhaustion status of the apheresis, final product and post-infusion samples for the T cell subsets can provide further insights.
Disclosures
Pulikkottil:Immuneel Therapeutics Private Limited: Current Employment. Kumar:Immuneel Therapeutics Private Limited: Current Employment. Soares:Immuneel Therapeutics Private Limited: Current Employment. Dhar:Immuneel Therapeutics Private Limited: Current Employment. Shetty:Immuneel Therapeutics Private Limited: Current Employment. T.I.:Immuneel Therapeutics Private Limited: Current Employment. Jakka:Immuneel Therapeutics Private Limited: Current Employment. Joseph:Immuneel Therapeutics Private Limited: Current Employment. Kumar MG:Immuneel Therapeutics Private Limited: Current Employment. Arasu:Immuneel Therapeutics Private Limited: Current Employment. Elluru:Immuneel Therapeutics Private Limited: Current Employment. Akheel:Immuneel Therapeutics Private Limited: Current Employment. Chenji:Immuneel Therapeutics Private Limited: Current Employment. Gandikota:Immuneel Therapeutics Private Limited: Current Employment. Nahar:Immuneel Therapeutics Private Limited: Current Employment. Kamat:Immuneel Therapeutics Private Limited: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal